- Global | English
- Search
Universal Cell Platform(TyUCell?)refers to the use of gene editing technology to transform allogeneic immune cells to eliminate immune rejection.
lEARN MORE TyUCell?The Quikin CAR-T Platform(Quikin CART) generates T cell products with gene knockout and stable integration of CAR cassette in one step through CRISPR gene editing technology.
lEARN MORE Quikin CARTEnhanced T cell Platforms(HyperTCell?), including HyperCART ? and HyperTCRT , are mainly to solve the global highlighted problem in solid tumor therapy through genetic modification of T cells.
lEARN MORE HyperTCell?
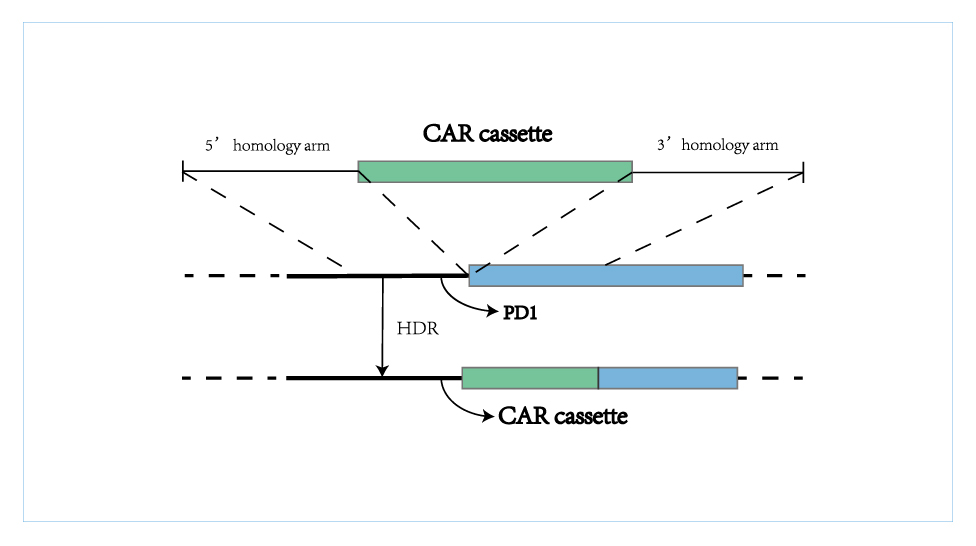
The Quikin CAR-T Platform (Quikin CART) generates T cell products with gene knockout and stable integration of CAR cassette in one step through CRISPR gene editing technology. As a new generation of CAR-T platform, it has several advantages, including simplified manufacturing process, shortened preparation time, higher product uniformity and avoiding the risk of tumorigenesis caused by random integration. The product with integration of anti-CD19 CAR cassette into PD1?locus combines PD1 immune checkpoint inhibition with CART anti-tumor activity. This platform has the potential to prepare enhanced CAR-T cells with gene knockout of multiple immune checkpoints, rapidly produce universal CAR-T cells and prepare dynamically regulated CAR-T cells with high safety, thus providing strong technical support for future improvement of CAR-T cells.